Introduction.
In the field of food science and technology, water is an important ingredient influencing taste, rheology and preservation of foods. Research on functional foods is currently popular; however, it is not yet well known that drinking water also has physiological functions, and that there are some health-beneficial effects of water (Shirahata, 2002, 2004). In the past decade, the decrease in the quality of tap water because of pollution of the global environment over time has become a major social problem. Air pollution affects water in soils, rivers, and farm products by acid rain. Chemicals in polluted water are considered to generate oxidative stress in the placenta of pregnant women, and this can cause various types of diseases in new-borns (Obolenskaya et al., 2010). The human body is approximately 60-80% water. The function of water in the body is mainly classified as follows. (1) The water molecule itself: flowing water affects cellular function and both development and functions of organs (Hirokawa, Tanaka, Okada, & Takeda, 2006; Hove et al., 2003), and hydration and Brownian movement of water are fundamentally important for protein function (Iwaki, Iwane, Shimokawa, Cooke, & Yanagida, 2009); (2) atoms and molecules derived from water molecules, such as protons (H+), hydrogen atoms (active hydrogen [H]), hydrogen anions (H-), hydrogen molecules (H2), oxygen molecules (O2), and reactive oxygen species (ROS); and molecules dissolved in water, such as mineral ions, mineral nanoparticles, organic and inorganic compounds, and gases. Functional water is activated water exhibiting specific functions. There are many activation methods such as electrolysis, treatment with a magnetic field, light irradiation, ultrasonication, bubbling with gases, strong water flow and collision, and treatment with some types of minerals or rocks. Functional water is defined by The Functional Water Association of Japan as water in which both treatment and function have been scientifically demonstrated or reproducible useful functions have been demonstrated among artificially treated waters. Among functional waters, electrolyzed water has been mostly investigated. Electrochemically reduced water (ERW) is produced near a cathode and electrochemically oxidized water (EOW) is produced near an anode. Potable ERW is a health-beneficial water as discussed here. EOW is also termed electrolyzed acidic water and is functional water exhibiting a sterilizing action, mainly due to hypochlorous acid, chlorine gas, and ozone (Bari, Sabina, Isobe, Uemura, & Isshiki, 2003) (Fig. 1A). Potable ERW (pH 8-10) is popular as a health beneficial water in Japan. ERW is also termed alkaline electrolyzed water, alkali-ionic water, alkaline cathodic water, and alkaline ionized water, based on its physicochemical and physiological aspects. ERW exhibits an alkaline pH, is hydrogen molecule-rich, and has a negative oxidation reduction potential (ORP) and reactive oxygen species (ROS)-scavenging activity (Shirahata et al., 2007). Studies on the functions of ERW were initiated in Japan in 1931, and its application to agriculture was first attempted in 1954. In 1960, it was applied to medical care as a health-beneficial water, and in 1966, the Ministry of Health, Labour and Welfare of Japan admitted that ERW was effective for chronic diarrhea, indigestion, abnormal gastrointestinal fermentation, antacid, and hyperacidity, and it authorized an ERW-producing device. In 1994, to mainly promote electrolyzed water use in society, the Functional Water Foundation was established with the support of the Ministry of Health, Labour and Welfare of Japan. Hayakawa (1999) reported that rats administrated alkali-ionic water for 8 weeks exhibited a significantly lower amount of total short chain fatty acids in the appendix than that in control rats; however, alkali ionic water did not affect the flora of intestinal bacteria. Rats administrated ERW of pH 10 had a more negative ORP in the intestine than that in control rats. A double-blind placebo-controlled study on the effects of alkali ionized water was performed using subjects who had abdominal symptoms such as pyrosis, dysphoria, abdominal distension, chronic diarrhea, and constipation from January 1996 to January 1999. The placebo control water was purified water obtained from tap water using an activated charcoal filter, which was then electrolyzed to obtain alkali-ionized water. The number of patients was 84 in the alkali-ionized water group and 79 in the purified water group. The patients drank at least 0.5 L of alkali-ionized water of pH 9.5 or purified water per day for 2 weeks. The results showed that alkali-ionized water significantly improved the abdominal complaints. In particular, chronic diarrhea patients who drank alkali-ionized water showed a significantly higher improvement efficacy of 94.1% comparedwith those who drank purified water (64.7%) (Tashiro, Kitahora, Fujiyama, & Banba, 2000). When the Drugs, Cosmetics and Medical Instruments Act of Japan was revised in 2005, a device for preparation of ERW was re-authorized based on considerable scientific evidence as a home managed medical device. The purpose of use was recognized to generate potable alkaline electrolyzed water for the improvement of gastrointestinal symptoms. The Japanese Society for Functional Water was established in 2001, and active studies on various functional waters including ERW have been performed to date. The biological effect of alkaline water consumption is object of controversy. The present paper presents a 3-year survival study on a population of 150 mice, and the data were analysed with accelerated failure time (AFT) model. Starting from the second year of life, nonparametric survival plots suggest that mice watered with alkaline water showed a better survival than control mice. Interestingly, statistical analysis revealed that alkaline water provides higher longevity in terms of “deceleration aging factor” as it increases the survival functions when compared with control group; namely, animals belonging to the population treated with alkaline water resulted in a longer lifespan. These results provide an informative and quantitative summary of survival data as a function of watering with alkaline water of long-lived mouse models. Biological effects of alkaline water were evaluated on a selected population of 150 mice (CD1, by Charles River, Oxford, UK). Pathogen-free mice were purchased and placed in a specific breeding facility. No other animal was present in the room. Contact with animal caretakers was minimized to feeding and watering. The population was divided into 3 groups, each consisting of 50 individuals, as follows:
- Group A: 50 mice conventionally fed and watered with alkaline water produced by the Water Ionizer (mod. NT010) by Asiagem (Italy). The Water Ionizer is a home treatment device for producing alkaline drinking water.
- Group B: 50 mice conventionally fed and watered with alkalized water obtained by dilution of a concentrated alkaline solution (AlkaWater by Asiagem, Italy). AlkaWater is a concentrated alkaline solution for preparing alkaline drinking water.
- Group C: 50 mice conventionally fed and watered as conventional (control group) with tap water. The local water supply was evaluated weekly for assuring the absence of toxins and pathogens. The pH values were in the 6.0–6.5 range.
All procedures involving animals were conducted in accordance with the Italian law on experimental animals and were approved by the Ethical Committee for Animal Experiments of the University of Padua and the Italian health Ministry (Aut. no. 39ter/2011). Efforts were made to minimize animal suffering. Result: The experiment consisted in an initial 15-day acclimatization period. After acclimatization, animals (50, group A) were watered with alkaline water (pH 8.5), obtained by the Water Ionizer (Asiagem, Italy), whereas group B animals (50) were watered with water alkalized at pH 8.5 by a concentrated alkaline solution (AlkaWater by Asiagem, Italy) for 15 days. Group C animals (50), control group, were watered with the local water supply. This period has been identified to gradually accustom the animals treated with alkaline water. At the end of the second period of acclimatization, group A and B animals were watered with alkaline water at pH 9.5 (by the Water Ionizer and by AlkaWater by Asiagem, Italy), while animals of group C were watered with local tap water.
Benefits of Alkaline water.
Alkaline water is water that is slightly basic. It contains basic minerals such as calcium, magnesium, or bicarbonate. These compounds bind to hydrogen ions in solution, making the water more basic. So, how do we get alkaline water if normal tap water is at about neutral pH? Natural alkaline water sources are usually springs, or a reservoir of natural water underneath the earth's surface. The rock structures holding the water may have basic minerals, such as calcium or limestone, that leak into the water, increasing the pH. Some companies, such as Poland Springs, have their own springs that they bottle water from. The Mammoth Hot Springs in Yellowstone National Park are examples of alkaline springs. Some companies add minerals to the water to make it more basic. Water can also be ionized, meaning it is broken up into hydrogen ions and hydroxide ions. The hydrogen ions are bound by minerals in the water, which makes the water more basic. However, not all ionized water has these minerals. Tap water that has been ionized will not have a lower pH, because there are no minerals to attach to the hydrogen ions. Alkaline water enhances the pH level of your drinking water, in contrast to RO water which makes it more acidic. As we've touched on already, RO removes all the minerals but this also has an adverse effect on the pH level.
Basic Information on pH of Water.
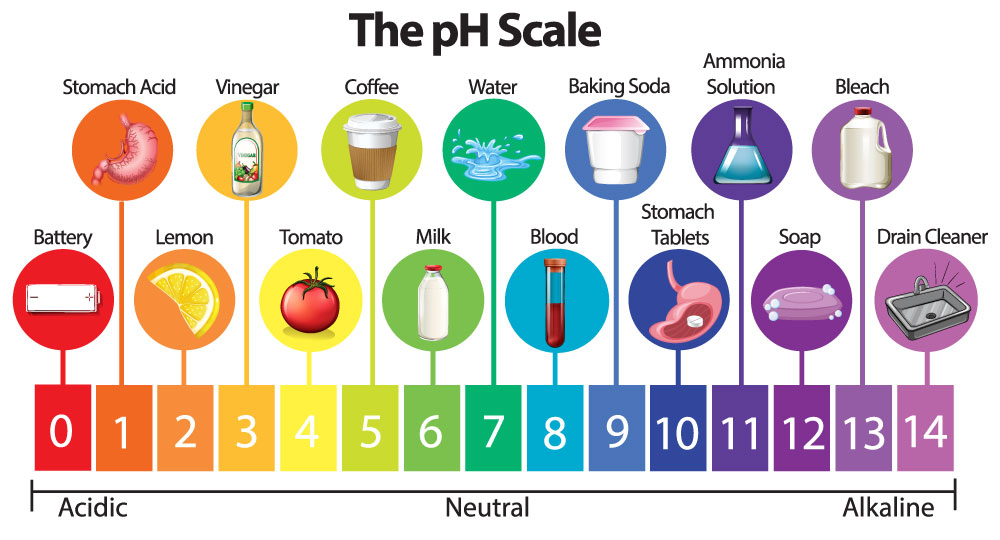
pH is a measure of how acidic/basic water is. The range goes from 0 to 14, with 7 being neutral. pH of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. Water that has more free hydrogen ions is acidic, whereas water that has more free hydroxyl ions is basic. Since pH can be affected by chemicals in the water, pH is an important indicator of water that is changing chemically. The pH of water determines the solubility (amount that can be dissolved in the water) and biological availability (amount that can be utilized by aquatic life) of chemical constituents such as nutrients (phosphorus, nitrogen, and carbon) and heavy metals (lead, copper, cadmium, etc.). For example, in addition to affecting how much and what form of phosphorus is most abundant in the water, pH also determines whether aquatic life can use it. In the case of heavy metals, the degree to which they are soluble determines their toxicity. Metals tend to be more toxic at lower pH because they are more soluble. So, in alkaline water this toxicity of heavy metals is absent because of its alkaline behaviour.